Understanding Briumvi: A Revolutionary MS Treatment
 Briumvi, known generically as ublituximab, is a monoclonal antibody that received FDA approval in December 2022 for treating relapsing forms of multiple sclerosis. Developed by TG Therapeutics, this innovative medication belongs to a class of drugs called anti-CD20 monoclonal antibodies, which specifically target B-cells that contribute to the inflammatory process in MS.
Briumvi, known generically as ublituximab, is a monoclonal antibody that received FDA approval in December 2022 for treating relapsing forms of multiple sclerosis. Developed by TG Therapeutics, this innovative medication belongs to a class of drugs called anti-CD20 monoclonal antibodies, which specifically target B-cells that contribute to the inflammatory process in MS.
What sets Briumvi apart from other MS treatments is its unique glycoengineered design, which enhances its ability to bind to immune cells and potentially provides superior efficacy compared to other anti-CD20 therapies. This advanced engineering allows for more precise targeting of the immune cells responsible for MS-related inflammation and damage.
The Mechanism of Action: How Briumvi Works
Multiple sclerosis occurs when the immune system mistakenly attacks the protective myelin sheath surrounding nerve fibers in the brain and spinal cord. B-cells, a type of white blood cell, play a crucial role in this autoimmune process by producing antibodies and inflammatory substances that contribute to myelin damage and neurological symptoms.
Briumvi works by binding to CD20 receptors on the surface of B-cells, marking them for destruction by the immune system. This targeted approach significantly reduces the number of circulating B-cells, thereby decreasing inflammation in the central nervous system and slowing the progression of MS-related damage.
The medication’s glycoengineered structure enhances its binding affinity to immune effector cells, potentially leading to more complete B-cell depletion compared to other anti-CD20 therapies. This improved mechanism may translate to better clinical outcomes and reduced disease activity for patients with relapsing MS.
Clinical Efficacy and Benefits
Clinical trials have demonstrated Briumvi’s impressive efficacy in treating relapsing forms of multiple sclerosis. The ULTIMATE I and II studies, which compared Briumvi to teriflunomide (Aubagio), showed superior results in reducing relapse rates and new brain lesions detected on MRI scans.
Patients treated with Briumvi experienced approximately 59% fewer relapses compared to those receiving teriflunomide. Additionally, MRI results showed significant reductions in new or enlarging T2 lesions and gadolinium-enhancing lesions, indicating decreased disease activity and inflammation in the brain.
The medication has also demonstrated benefits in slowing disability progression, helping patients maintain their functional abilities and quality of life. Many patients report improved stability in their symptoms and reduced frequency of MS relapses, allowing them to better manage their daily activities and long-term planning.
Administration and Treatment Protocol
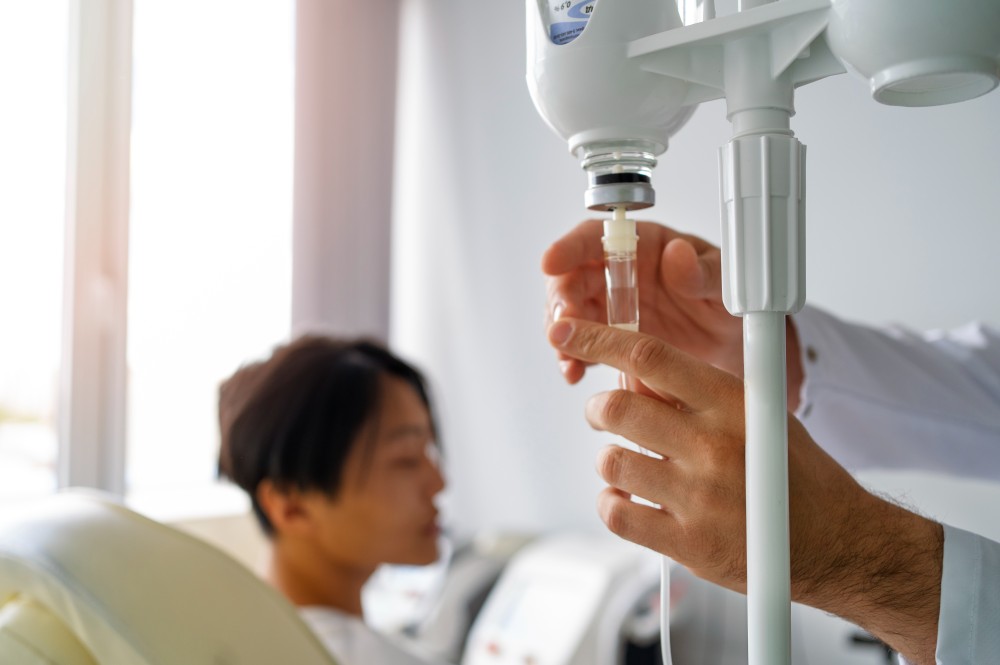
Briumvi is administered as an intravenous infusion in our clinic setting, ensuring proper medical supervision and immediate access to emergency care if needed. The treatment schedule consists of two initial doses given two weeks apart, followed by maintenance doses every six months.
The initial doses are typically administered over approximately four hours, while subsequent maintenance infusions may be completed more quickly as patients develop tolerance to the medication. Our experienced infusion team closely monitors patients throughout each treatment session, checking vital signs and watching for any adverse reactions.
Before each infusion, patients receive premedication with corticosteroids and antihistamines to minimize the risk of infusion-related reactions. This comprehensive approach to administration helps ensure patient safety and comfort throughout the treatment process.
Patient Selection and Evaluation
Not all patients with multiple sclerosis are candidates for Briumvi treatment. Our comprehensive evaluation process includes assessing disease type, activity level, and previous treatment responses. The medication is specifically approved for relapsing forms of MS, including relapsing-remitting MS and active secondary progressive MS.
Candidates undergo thorough screening, including complete blood counts, liver function tests, and screening for hepatitis B and C infections. We also evaluate patients for any active infections or immunodeficiencies that might contraindicate B-cell depleting therapy.
Our neurological team reviews each patient’s medical history, current medications, and individual risk factors to determine whether Briumvi is the most appropriate treatment option. This personalized approach ensures that patients receive the most suitable therapy for their specific circumstances.
Safety Profile and Monitoring
Like all disease-modifying therapies for MS, Briumvi carries potential risks that require careful monitoring and management. The most common side effects include infusion-related reactions, upper respiratory tract infections, and urinary tract infections. More serious but less common risks include severe infections and reactivation of hepatitis B virus.
Our clinic implements rigorous safety protocols, including regular monitoring of blood counts, liver function, and immunoglobulin levels. We also screen for signs of progressive multifocal leukoencephalopathy (PML), a rare but serious brain infection that can occur with immunosuppressive treatments.
Patients are educated about recognizing signs of infection and when to seek immediate medical attention. We maintain close communication with patients between infusions to monitor for any concerning symptoms and provide prompt intervention when needed.
Managing Infusion-Related Reactions
Infusion-related reactions are among the most common side effects of Briumvi treatment, typically occurring during or shortly after the first infusion. These reactions may include fever, chills, fatigue, headache, and muscle pain. Our experienced infusion team is well-prepared to manage these reactions and ensure patient comfort.
We use evidence-based premedication protocols to minimize the risk of infusion reactions and have established procedures for managing any reactions that do occur. Most reactions are mild to moderate and resolve quickly with appropriate intervention, allowing patients to continue their treatment successfully.
Long-term Treatment Considerations
Briumvi treatment requires long-term commitment and regular monitoring to ensure optimal outcomes. The medication’s effects on B-cells can persist for several months after each infusion, providing sustained protection against MS relapses and disease progression.
Our clinic provides comprehensive long-term care coordination, including regular neurological assessments, MRI monitoring, and coordination with other healthcare providers. We work closely with patients to develop individualized management plans that address their evolving needs and treatment goals.
Integration with Comprehensive MS Care
Briumvi treatment is most effective when integrated into a comprehensive multiple sclerosis management program. Our clinic offers a multidisciplinary approach that includes neurology, nursing, pharmacy, and rehabilitation services to address all aspects of MS care.
We provide patient education about MS management, symptom monitoring, and lifestyle modifications that can complement medical treatment. Our team also offers resources for managing fatigue, cognitive changes, and other MS-related symptoms that may persist despite disease-modifying therapy.
Conclusion
Briumvi represents a significant advancement in multiple sclerosis treatment, offering superior efficacy compared to traditional oral therapies and a convenient dosing schedule that may improve patient adherence. Its innovative glycoengineered design and proven clinical benefits make it an valuable option for patients with relapsing forms of MS.
At our neurological clinic, we are committed to providing cutting-edge MS care that incorporates the latest therapeutic advances. Our experienced team works collaboratively with each patient to develop personalized treatment plans that optimize outcomes while minimizing risks, ensuring that patients receive the highest quality care for their multiple sclerosis journey.
Learn about complementary therapies and lifestyle strategies that can enhance your MS treatment outcomes by exploring our patient resources section on comprehensive multiple sclerosis management. Visit https://americaninfusioncare.com/ to know more.
